Tregs and beyond: discover how SR-TIGET scientists are engineering peripheral immune tolerance for tomorrow’s precision therapies.

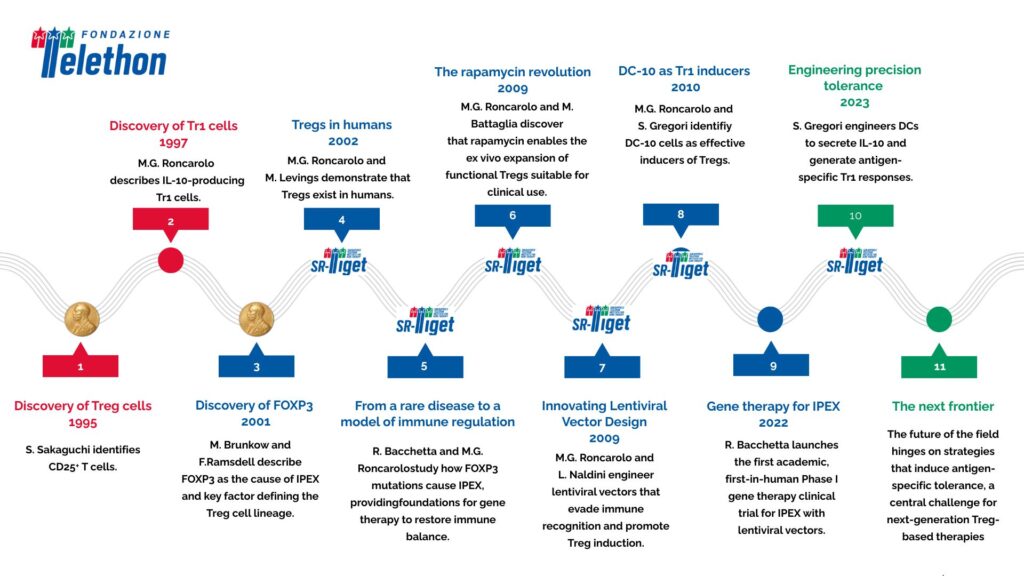
The discovery of regulatory T cells (Tregs) and type 1 regulatory T cells (Tr1) in the 1990s marked a turning point in immunology, paving the way for the concept of peripheral immune tolerance. Three decades later, the pioneering vision of scientists at the San Raffaele–Telethon Institute for Gene Therapy (SR-TIGET) continues to guide the evolution of these discoveries toward clinical translation.
Today, the goal is no longer simply to understand immune tolerance — it is to engineer it. From ex vivo expanded Tregs and Tr1 cells to in vivo reprogramming and gene-edited cell products, the field is entering a new era of precision therapies.
DC-10 cells and the first Tr1-based clinical trials
Within the field of peripheral immune tolerance, one advancement achieved by SR-TIGET is the identification by Silvia Gregori, now Group Leader of the Mechanisms of Peripheral Tolerance Unit at SR-TIGET, of other important players in this process: a subset of myeloid regulatory cells, known as DC-10, capable of producing the molecule IL-10. DC-10, which are present in vivo and can be generated in vitro, are effective inducers of Tregs and are currently used to generate Tr1 cells in clinical settings.
A Phase I clinical trial (NCT03198234) recently evaluated the efficacy of a Tr1-enriched cell therapy generated with DC-10 in preventing graft-versus-host disease (GvHD) following allogeneic hematopoietic stem cell transplantation. The early results were encouraging: Tr1 cells proved to be the active, antigen-specific component responsible for suppressing GvHD, with persistence in patients up to one year post-treatment. This study offered clinical evidence supporting the potential of regulatory cell therapy to promote immune tolerance, marking an important step in translational immunology.
Engineering tolerance through gene and cell therapies
Building on this, Gregori’s team is working to combine tolerogenic dendritic cells and lentiviral vector technology to achieve antigen-specific tolerance in vivo. Gregori’s group recently developed an approach that uses lentiviral vectors to engineer DCs with the ability to secrete IL-10 and present selected antigens. These engineered DCs can instruct the immune system to generate antigen-specific Tr1 cells, potentially restoring tolerance in autoimmune and inflammatory diseases.
This strategy reflects a broader trend in the field — moving from broad immunosuppression to precision immunomodulation, where the goal is to control only the harmful immune reactions while preserving protective responses.
From the lab to the biotech landscape
The translational potential of Treg and Tr1-based therapies has given rise to a rapidly expanding biotech ecosystem. Today, more than 30 biotechnology companies worldwide are developing therapies to induce immune tolerance using regulatory cells. Among them is Tr1xBio, founded by Maria Grazia Roncarolo, which focuses on advancing Tr1-based cell therapies for autoimmune diseases and transplantation.
At the same time, several companies are exploring in vivo induction of Tregs using small molecules and biologics such as IL-2 and rapamycin, aiming to simplify production and make these therapies more accessible. These in vivo strategies might offer a more scalable alternative to traditional adoptive Treg cell-based therapies.
The next frontier: CAR-Tregs and next-generation precision therapies
Over the past fifteen years, numerous clinical trials — mostly conducted in academic settings — have tested the safety and biological activity of regulatory T cells (Tregs) in a wide range of conditions, from organ transplantation and graft-versus-host disease (GvHD) to autoimmune and inflammatory disorders. The results have shown that Treg-based therapies are both feasible and generally safe, offering a promising way to restore immune balance in diseases where the immune system mistakenly attacks the body’s own tissues. However, researchers continue to monitor patients over the long term to better understand any potential risks associated with sustained immune modulation.
Building on these advances, the field is now moving toward more precise and targeted approaches. Because Tregs act locally within tissues, antigen-specific Tregs — designed to recognise and regulate immune responses against a defined molecular target — are believed to be safer and more effective than polyclonal Tregs, which act more broadly. To achieve this goal, scientists are developing genetically engineered Tregs, equipped with CARs (chimeric antigen receptors) or TCRs (T-cell receptors) that allow them to target disease-specific antigens. This strategy, inspired by the success of CAR-T cells in oncology, marks a new frontier in precision immunomodulation.
A future rooted in discovery
From the discovery of IL-10–producing Tr1 cells to the development of gene and cell therapies for IPEX, from the use of rapamycin to expand regulatory T cells to the design of innovative strategies for inducing immune tolerance in vivo, SR-TIGET has played a defining role in bridging fundamental immunology and clinical translation.
The 2025 Nobel Prize for the discovery of peripheral immune tolerance also reflects this enduring legacy — a story shaped by the pioneering contributions of Maria Grazia Roncarolo, Rosa Bacchetta, Manuela Battaglia, and Silvia Gregori, whose work at SR-TIGET continues to influence the field of immunology and inspire the next generation of innovative therapies.
References
- Chen PP, Cepika AM, Agarwal-Hashmi R, Saini G, Uyeda MJ, Louis DM et al. Alloantigen-specific type 1 regulatory T cells suppress through CTLA-4 and PD-1 pathways and persist long-term in patients. Sci Transl Med. 2021 Oct 27;13(617):eabf5264. doi: 10.1126/scitranslmed.abf5264. Epub 2021 Oct 27. PMID: 34705520; PMCID: PMC9451143.
- Passeri L, Andolfi G, Bassi V, Russo F, Giacomini G, Laudisa C et al. Tolerogenic IL-10-engineered dendritic cell-based therapy to restore antigen-specific tolerance in T cell mediated diseases. J Autoimmun. 2023 Jul;138:103051. doi: 10.1016/j.jaut.2023.103051. Epub 2023 May 22. PMID: 37224733.